Lime production is an age-old industrial process that transforms natural limestone into quicklime (calcium oxide) or hydrated lime (calcium hydroxide), materials used in a wide range of industries, from construction to environmental management. Understanding the production of lime requires an exploration of its raw materials, the chemical reactions involved, and the technological processes used to create this essential substance.
1. Raw Materials: Limestone
Lime production begins with limestone, a sedimentary rock primarily composed of calcium carbonate (CaCO₃). Limestone deposits are found in vast quantities worldwide, and this rock is the foundation of lime manufacturing. In nature, limestone forms through the accumulation of shells, coral, and other organic materials, often in marine environments.
To produce lime, limestone must first be extracted through mining, either by quarrying (in shallow deposits) or underground mining (in deeper reserves). The purity of limestone can vary, and higher purity limestone results in higher-quality lime.
2. Crushing and Screening
After extraction, the limestone is transported to a processing facility, where it undergoes several mechanical processes. First, it is crushed into smaller chunks or aggregates to increase its surface area, which facilitates the subsequent chemical reactions. The size of the crushed limestone depends on the type of kiln being used in the next stage.
Screening is also an essential part of this step. Large particles are separated from smaller ones, ensuring that only the appropriate size limestone enters the kiln.
3. Calcination: Turning Limestone into Lime
The core process of lime production is calcination, which involves heating limestone to high temperatures (typically between 900°C and 1000°C) in a kiln. This process breaks down the calcium carbonate in the limestone into quicklime (calcium oxide) and carbon dioxide (CO₂), a chemical reaction represented as:
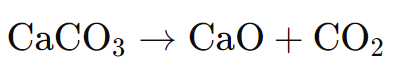
The carbon dioxide gas escapes into the atmosphere, and the remaining solid calcium oxide is referred to as quicklime. The temperature in the kiln must be carefully controlled to ensure that the calcination process is efficient while minimizing the production of unwanted byproducts.
There are several types of kilns used for calcination, including:
Vertical Shaft Kilns (VSKs): These are tall, vertical kilns in which limestone is heated from below. The heat is provided by burning fuel such as coal or natural gas.
Rotary Kilns: These large, cylindrical kilns rotate as limestone is fed in one end and moves through the kiln, getting progressively hotter as it travels. Rotary kilns are known for their efficiency in handling large quantities of material.
Beehive Kilns: Used in traditional lime production, beehive kilns are masonry kilns with a dome-like shape.
The choice of kiln depends on the scale of production, energy efficiency, and desired product characteristics.
4. Cooling and Screening of Quicklime
Once the limestone has been converted into quicklime, it needs to cool down before it can be further processed or used. Quicklime is extremely hot when it exits the kiln, and cooling is an important step to ensure that the lime does not react unpredictably when exposed to water or air. Cooling is typically done using air or water.
Once cooled, the quicklime is often screened to separate finer particles from larger chunks. The particle size affects the reactivity of the quicklime, with finer particles being more reactive and faster in their chemical reactions.
5. Hydration: Turning Quicklime into Hydrated Lime (Optional)
For certain applications, quicklime is further processed to produce hydrated lime (calcium hydroxide). This involves adding water to quicklime in a controlled process known as hydration. The chemical reaction is:
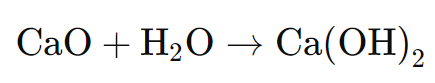
The result is a fine, dry powder known as hydrated lime. Hydrated lime is used in applications where a finer product is needed, such as in water treatment, environmental control (e.g., flue gas desulfurization), and construction materials.
The hydration process can take place in different types of equipment, including slaking tanks and hydrators, where quicklime is slowly mixed with water to control the heat and prevent excessive splattering or reaction.
6. Quality Control and Packaging
Once the lime is produced, whether as quicklime or hydrated lime, it undergoes quality control testing. Lime is tested for purity, particle size distribution, and reactivity to ensure that it meets the standards for its intended use. This stage involves both laboratory testing and on-site inspection.
Finally, the lime is packaged into bulk containers, bags, or transported by conveyor to be distributed for commercial or industrial use.
Industrial Applications of Lime
Lime plays a crucial role in various industries, with applications ranging from environmental control to manufacturing. Some of the primary uses include:
Construction: Lime is used in the production of cement, mortar, and concrete.
Environmental Management: Lime is widely used in water treatment, neutralizing acidic water and treating sewage sludge.
Steel Manufacturing: Lime is used as a flux in the production of steel to remove impurities.
Chemical Industry: Lime serves as a raw material for producing a variety of chemicals, including calcium carbide and soda ash.
Agriculture: Lime is used to adjust soil pH, improving crop yield.
Conclusion
The production of lime is a process that involves several intricate steps, from the extraction of limestone to the calcination process in kilns. It is a vital industrial process that has been fundamental to human progress for centuries. Lime's versatility and importance in industries such as construction, steel, and environmental management make it one of the most significant materials in modern industry.

 English
English 中文简体
中文简体 русский
русский Français
Français Español
Español عربى
عربى
